Research in the group falls within the field of small molecule activation - using transition metal and main group species to increase the reactivity of small, inert molecules. Our focus is on studying homogeneous systems, and developing new methodologies for the reduction of molecules such as dinitrogen and carbon dioxide.
Frustrated Lewis Pair Chemistry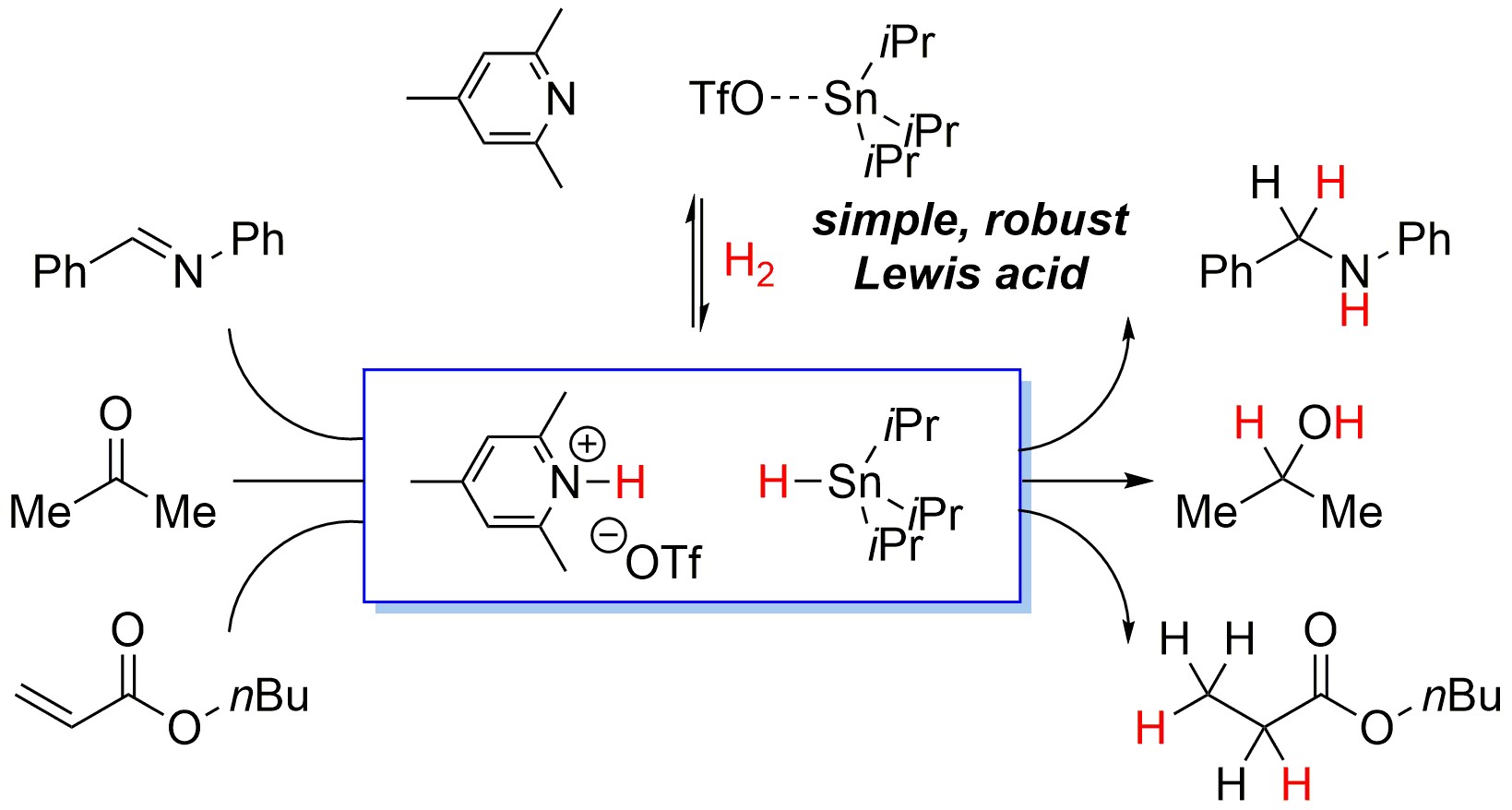
Catalysts for hydrogenation reactions typically utilise transition metals, but their toxicity, scarcity and cost are often significant problems. Frustrated Lewis pairs (FLPs) are combinations of Lewis acids and Lewis bases, both possessing bulky substituents, that for steric reasons do not form a classical adduct. These systems are mainly formed from main group species and often are capable of activating H2, essentially splitting it into H+ and H- equivalents, and allowing for the possibility of transition-metal free hydrogenation. Of particular interest is the catalytic hydrogenation of organic functional groups (imines, ketones, alkenes), as well as small molecules (CO2, CO) to liquid fuels. Whilst much of the group’s research has focused on boron-centred Lewis acids, our recent publication in Angewandte Chemie International Edition detailed the first catalytic, moisture-tolerant, FLP-mediated hydrogenations using a Sn-based Lewis acid - the paper can be found here.
Our recent work has expanded these ideas to achieve moisture tolerant methodologies for FLP-catalysed hydrogenations (here) and apply FLP mechanisms to low-valent Sn compounds (here).
Dinitrogen Reduction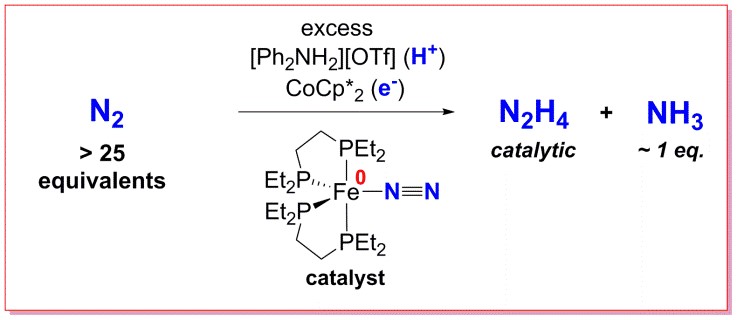
Dinitrogen, N2, is one of the most inert molecules known, and its catalytic reduction to products such as NH3 and N2H4 remains a fundamental chemical challenge, as well as an industrially important one. Currently, the Haber process produces all synthetic ammonia that humans use for fertiliser to improve agricultural yields and hence help feed the world's population. However, it does so at an immense energy cost (∼2% global energy demand) due to operation at high temperatures and pressures. Since iron is used in this process, and an iron-only nitrogenase has been discovered in nature, iron dinitrogen complexes are the focus of our research in this area.
A notable success in the group has been the catalytic formation of N2H4 from N2 using a simple iron phosphine complex - the paper detailing this has been published in the Journal of the American Chemical Society and can be found here. We have also recently used silyl cations as models of H+ during the reduction of nitrogen with these compounds, in order to isolate and characterise reactive intermediates (here).